研究業績
ORCID orcid.org/0000-0002-0309-9507
論文(責任著者)@新潟大学
| 47 | Paper Ring-Strain Effects in Sommelet-Hauser Rearrangement of Five- and Six-Membered Fused Bicyclic Ammonium Ylides Tayama, E.; Itoyama, K.; Sasazaki, J. Synthesis 2026, 58, 95-106. 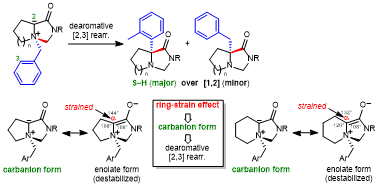 DOI: 10.1055/a-2707-7636 |
| 46 | Full Review Nitrogen- and Sulfur-Based Stevens and Related Rearrangements Tayama, E. In Comprehensive Organic Synthesis, 3rd Ed.,Vol. 3, Chap. 4.14; Molander, G.; Knochel, P., Ed.; Elsevier: 2025, 677-747. 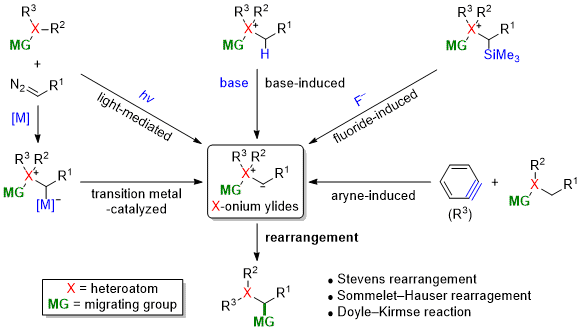 DOI: 10.1016/B978-0-323-96025-0.00029-6 |
| 45 | Research Article Controlling the position of the nucleophilic ring-opening of 2-EWG-substituted azetidinium salts with fluoride by the N-1-(1-naphthyl)ethyl substituent and BINAM-derived bis-urea organocatalyst Tayama, E.; Tsutsumi, R.; Uraguchi, D. Tetrahedron 2024, 167, 134274. 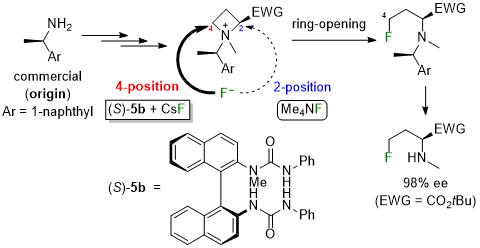 DOI: 10.1016/j.tet.2024.134274 |
| 44 | Research Article Synthesis of α-Fluoro-γ-aminobutyric Acid Derivatives by Nucleophilic Ring-opening of Azetidine-2-carboxylic Acid-derived Ammonium Salts with Fluoride Tayama, E.; Kurosaki, Y.; Iida, M.; Aida, T.; Nakanome, N. Tetrahedron 2023, 142, 133528. 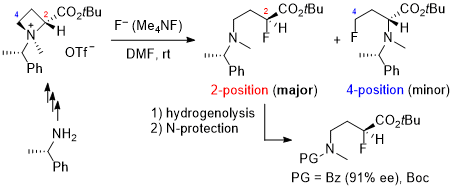 DOI: 10.1016/j.tet.2023.133528 |
| 43 | Short Review Recent Advances in the Generation of Onium Ylides for Sommelet-Hauser Rearrangements Tayama, E. Synthesis 2022, 54, 5395. 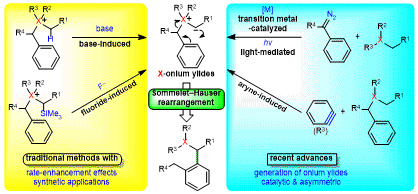 DOI: 10.1055/a-1914-7261 |
| 42 | Research Article Chiral Tetraalkynylborate as a Chiral Solvating Agent for N-Chiral Tetraalkylammonium Salts Tayama, E.; Nishio, R. Tetrahedron 2022, 114, 172783. 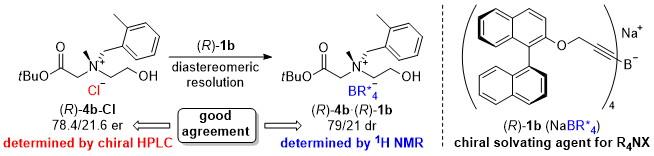 DOI: 10.1016/j.tet.2022.132783 |
| 41 | Research Article Base-Induced Sommelet–Hauser Rearrangement of N-(Pyridinylmethyl) Tetraalkylammonium Salts Tayama, E.; Shimizu, G.; Nakao R. Tetrahedron 2022, 111, 132721. 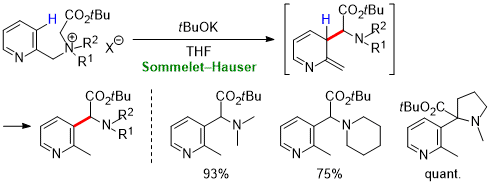 DOI: 10.1016/j.tet.2022.132721 |
| 40 | Synthesis of Tertiary Alkyl Fluorides and Chlorides by Site-Selective Nucleophilic
Ring-Opening Reaction of α-Aryl Azetidinium Salts Tayama, E.; Kawai, K. RSC Adv. 2021, 11, 39607-39618.. 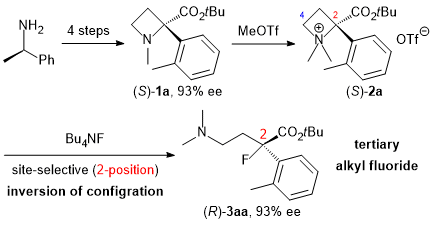 DOI: 10.1039/D1RA08706A ※オープンアクセス |
| 39 | Synthesis of Optically Active 2-Substituted Azetidine-2-carbonitriles from Chiral 1-Arylethylamine via α-Alkylation of N-Borane Complexes Tayama, E.; Nakanome, N. RSC Adv. 2021, 11, 23825-23837. 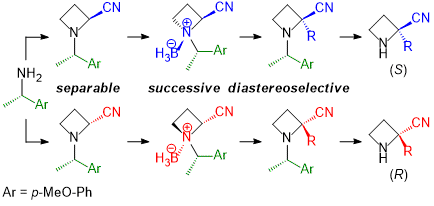 DOI: 10.1039/D1RA04585G ※オープンアクセス |
| 38 | Brønsted Acid-Catalyzed Aza-Ferrier Reaction of N,O-Allenyl Acetals: Synthesis
of β-Amino-α-methylene Aldehydes Tayama, E.; Ishikawa, Y. J. Org. Chem. 2020, 85, 9405-9414. 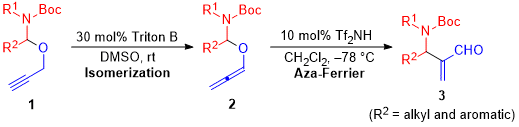 DOI: 10.1021/acs.joc.0c01047 |
| 37 | Base-induced Sommelet-Hauser Rearrangement of N-(α-(2-Oxyethyl)branched)benzylic
Glycine Ester-derived Ammonium Salts via a Chelated Intermediate Tayama, E.; Hirano, K.; Baba, S. Tetrahedron 2020, 76, 131064. 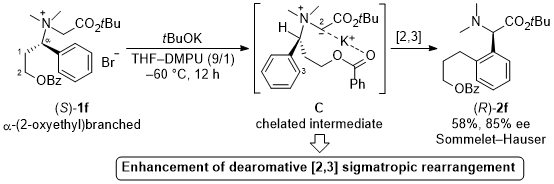 DOI: 10.1016/j.tet.2020.131064 |
| 36 | Base-promoted Aromatic [3,3] Sigmatropic Rearrangement of N-Acyl-O-arylhydroxylamine
Derivatives Tayama, E.; Hirano, K. Tetrahedron 2019, 75, 665-673.  DOI: 10.1016/j.tet.2018.12.058 |
| 35 | Chiral Tetraaryl- and Tetraalkynylborates as Chiral Solvating Agents for
Tetraalkylammonium Salts Tayama, E.; Sugawara, T. Eur. J. Org. Chem. 2019, 803-811. 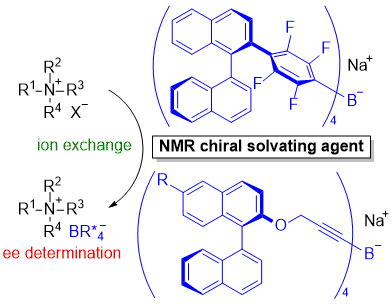 DOI: 10.1002/ejoc.201801448 |
| 34 | Base-promoted Diastereoselective α-Alkylation of Borane N-((S)-1’-Phenylethyl)azetidine-2-carboxylic
acid Ester Complexes Tayama, E.; Nishio, R.; Kobayashi, Y. Org. & Biomol. Chem. 2018, 16, 5833-5845. 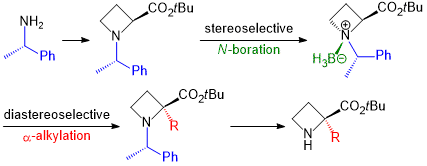 DOI: 10.1039/C8OB01395K |
| 33 | Dearomative [2,3] Sigmatropic Rearrangement of Ammonium Ylides Followed
by 1,4-Elimination to Form α-(ortho-Vinylphenyl)Amino Acid Esters Tayama, E.; Sotome, S. Org. & Biomol. Chem. 2018, 16, 4833-4839. 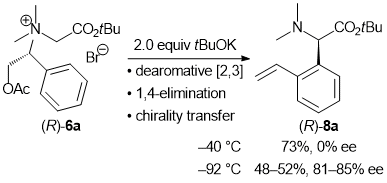 DOI: 10.1039/C8OB01091A |
| 32 | Structural and Mechanistic Studies of the Base-Induced Sommelet-Hauser
Rearrangement of N-α-Branched Benzylic Azetidine-2-carboxylic Acid-Derived
Ammonium Salts Tayama, E.; Watanabe, K.; Sotome, S. Org. & Biomol. Chem. 2017, 15, 6668-6678. 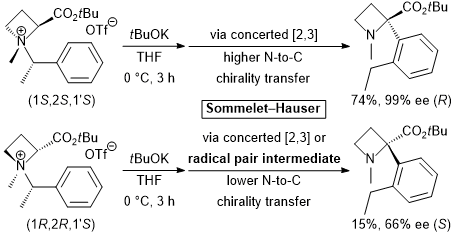 DOI: 10.1039/C7OB01391D |
| 31 | Diaza [1,4] Wittig-Type Rearrangement of N-Allylic-N-Boc-Hydrazines into
γ-Amino-N-Boc-Enamines Tayama, E.; Kobayashi, Y.; Toma, Y. Chem. Commun. 2016, 52, 10570-10573. 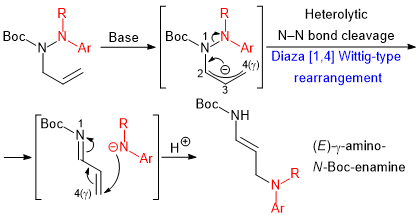 DOI: 10.1039/C6CC04626F |
| 30 | Ring-Strain Effect in Base-Induced Sommelet-Hauser Rearrangement: Application
to Successive Stereo-Controlled Transformations Tayama, E.; Watanabe, K.; Matano, Y. Eur. J. Org. Chem. 2016, 3631-3641. 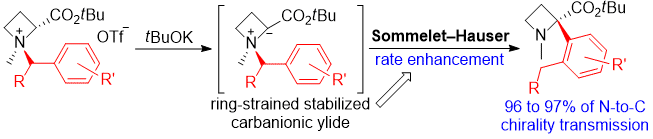 DOI: 10.1002/ejoc.201600611 |
| 29 | Review Ring-Substitution, Enlargement, and Contraction by Base-Induced Rearrangements of N-Heterocyclic Ammonium Salts Tayama, E. Heterocycles 2016, 92, 793-828. 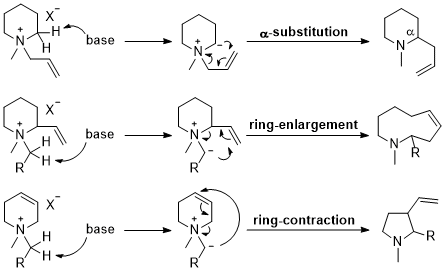 DOI: 10.3987/REV-16-837 |
| 28 | Regioselective Synthesis of Secondary 1,3-Dienamides by Successive Eliminations Tayama, E.; Saito, S. Tetrahedron 2016, 72, 599-604. 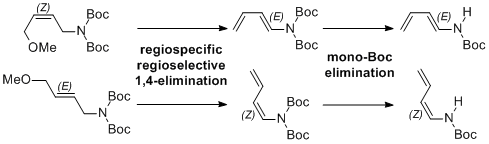 DOI: 10.1016/j.tet.2015.11.065 |
| 27 | Personal Account Recent Advances in the Base-Induced Sommelet-Hauser Rearrangement of Amino Acid-Derived Ammonium Ylides Tayama, E. Chem. Rec. 2015, 789-800. 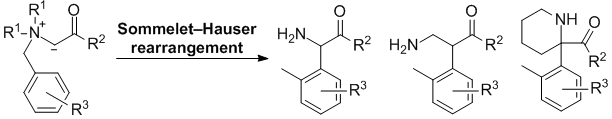 DOI: 10.1002/tcr.201500009 |
| 26 | Copper-Catalyzed Regiospecific and 1,2-Regioselective Cyclopropanation
of (1Z)-1-Amino- and (1Z)-1-Oxy-1,3-Butadienyl Derivatives Tayama, E.; Saito, S. Synlett 2015, 1880-1884. 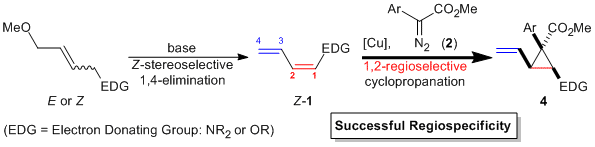 DOI: 10.1055/s-0034-1380426 |
| 25 | Stereoselective Preparation of (1Z)- and (1E)-N-Boc-1-Amino-1,3-Dienes
by Stereospecific Base-Promoted 1,4-Elimination Tayama, E.; Toma, Y. Tetrahedron 2015, 71, 554-559. 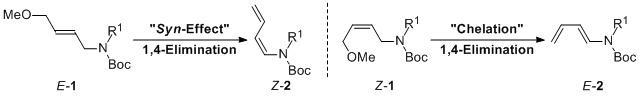 DOI: 10.1016/j.tet.2014.12.039 |
| 24 | Double Axial Chirality Promoted Asymmetric [2,3] Stevens Rearrangement
of N-Cinnamyl L-Alanine Amide-Derived Ammonium Ylides Tayama, E.; Naganuma, N.; Iwamoto, H.; Hasegawa, E. Chem. Commun. 2014, 50, 6860-6862. 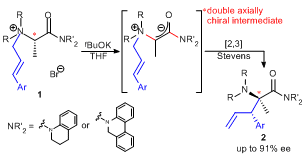 DOI: 10.1039/C4CC02536A |
| 23 | Copper(II)-Acid Catalyzed Cyclopropanation of 1,3-Dienamides by Electrophilic
Activation of α-Aryl Diazoesters Tayama, E.; Horikawa, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2014, 55, 3041-3044. 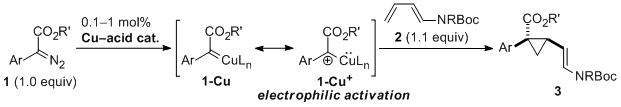 DOI: 10.1016/j.tetlet.2014.03.115 |
| 22 | 総合論文 第四級アンモニウムイリドを中間体とする塩基促進型Sommelet-Hauser 田山 英治 有機合成化学協会誌, 2014, 72, 418-428. 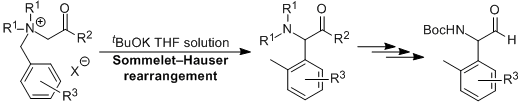 DOI: 10.5059/yukigoseikyokaishi.72.418 |
| 21 | 1,4-Elimination/Bronsted Acid Catalyzed Aza-Ferrier Reaction Sequence as
an Entry to β-Amino-β,γ-Unsaturated Aldehydes Tayama, E.; Horikawa, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron 2013, 69, 2745-2752. 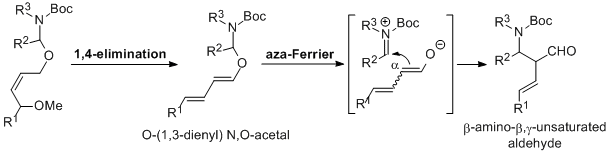 DOI: 10.1016/j.tet.2013.01.088 |
| 20 | A Unique and Simple Preparative Method for α-Arylpipecolinic Acid Esters
via Base-induced Sommelet-Hauser Rearrangement Tayama, E.; Sato, R.; Ito, M.; Iwamoto, H.; Hasegawa, E. Heterocycles 2013, 87, 381-388. 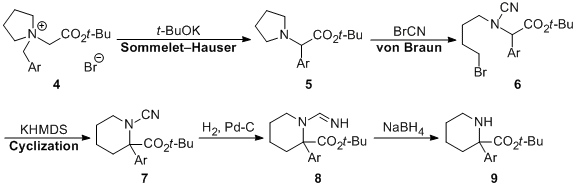 DOI: 10.3987/COM-12-12619 |
| 19 | Copper(II)-Acid Co-Catalyzed Intermolecular Substitution of Electron-Rich Aromatics with Diazoesters Tayama, E.; Ishikawa, M.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2012, 53, 5159-5161. 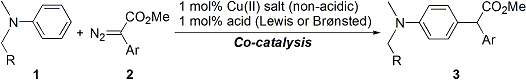 DOI: 10.1016/j.tetlet.2012.07.070 |
| 18 | A Formal Method for the De-N,N-Dialkylation of Sommelet-Hauser Rearrangement Products Tayama, E.; Sato, R.; Takedachi, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron 2012, 68, 4710-4718. 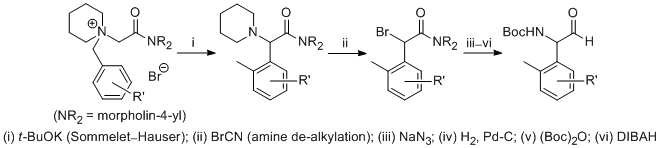 DOI: 10.1016/j.tet.2012.04.015 |
| 17 | 1,2-Dimethoxy-4,5-dimethylene: A New Protecting Group for Acyclic Amino Acid Derivatives Prepared by Stevens Rearrangement Tayama, E.; Takedachi, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2012, 53, 1373-1375. 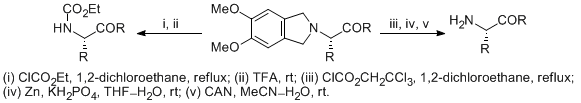 DOI: 10.1016/j.tetlet.2012.01.013 |
| 16 | Asymmetric α-2-Tosylethenylation of N,N-Dialkyl-L-Amino Acid Esters via the Formation of Non-Racemic Ammonium Enolates Tayama, E.; Igarashi, T.; Iwamoto, H.; Hasegawa, E. Org. Biomol. Chem. 2012, 10, 339-345. 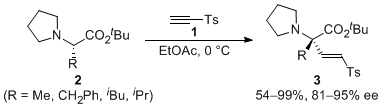 DOI: 10.1039/C1OB06074K |
| 15 | Asymmetric α-2-Tosylvinylation of in situ-generated N-2-Tosylvinyl Proline-Derived
Ammonium Ylides Igarashi, T.; Tayama, E.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2011, 52, 1819-1821. 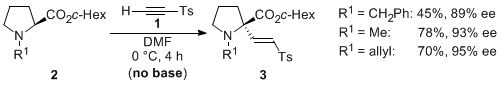 DOI: 10.1016/j.tetlet.2011.02.040 |
| 14 | Copper(II) Triflate Catalyzed Intermolecular Aromatic Substitution of N,N-Disubstituted
Anilines with Diazoesters Tayama, E.; Yanaki, T.; Iwamoto, H.; Hasegawa, E. Eur. J. Org. Chem. 2010, 6719-6721. 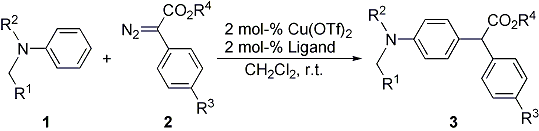 DOI: 10.1002/ejoc.201001083 |
| 13 | Remarkable Enhancement Effect of Potassium tert-Butoxide/THF Solution in
Base-Induced Sommelet-Hauser Rearrangements Tayama, E.; Takedachi, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron 2010, 66, 9389-9395. 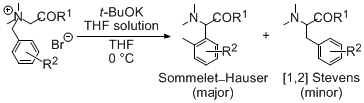 DOI: 10.1016/j.tet.2010.09.105 |
| 12 | Resolution of Nitrogen-Centered Chiral Tetraalkylammonium Salts: Application
to [1,2] Stevens Rearrangements with N-to-C Chirality Transmission Tayama, E.; Otoyama, S.; Tanaka, H. Tetrahedron: Asymmetry 2009, 20, 2600-2608. 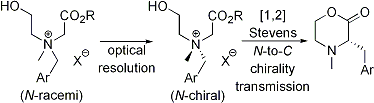 DOI: 10.1016/j.tetasy.2009.10.025 |
| 11 | New Synthetic Routes to Optically Active α-Quaternary α-Aryl Amino Acid Derivatives via the Diastereoselective Stevens and Sommelet-Hauser Rearrangements Tayama, E.; Orihara, K.; Kimura, H. Org. & Biomol. Chem. 2008, 6, 3673-3680. 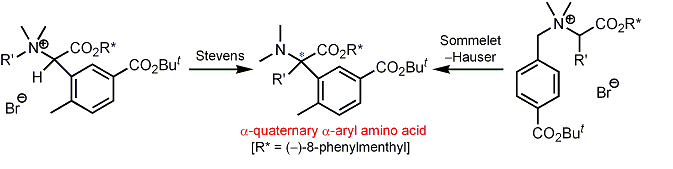 DOI: 10.1039/b811162f |
| 10 | Bronsted Acid Catalyzed Regioselective Aza-Ferrier Reaction: A Novel Synthetic
Method of α-(N-Boc-2-pyrrolidinyl)aldehydes Tayama, E.; Otoyama, S.; Isaka W. Chem. Comm. 2008, 4216-4218. 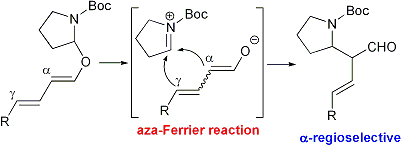 DOI: 10.1039/b806492j |
| 9 | Asymmetric Sommelet-Hauser Rearrangement of N-Benzylic Ammonium Salts Tayama, E.; Kimura, H. Angew. Chem. Int. Ed. 2007, 46, 8869-8871. 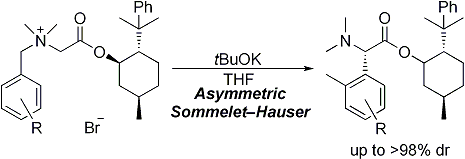 DOI: 10.1002/anie.200703832 |
| 8 | A Facile Synthetic Method of α-Quaternary-β,γ-Unsaturated Aldehydes via the Stereoselective 1,4-Elimination and α-Regioselective Ferrier Reaction Tayama, E.; Hashimoto, R. Tetrahedron Lett. 2007, 48, 7950-7952. 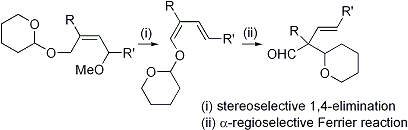 DOI: 10.1016/j.tetlet.2007.09.049 |
| 7 | A Facile Method for the Stereoselective Preparation of (1E, 3E)-4-Substituted-1-Amino-1,3-Dienes via 1,4-Elimination Tayama, E.; Sugai, S. Tetrahedron Lett. 2007, 48, 6163-6166. 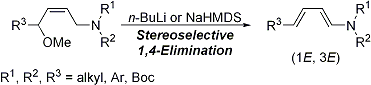 DOI: 10.1016/j.tetlet.2007.06.140 |
| 6 | An Efficient Optical Resolution of Nitrogen-Centered Chiral β-Hydroxy-Tetraalkylammonium
Salts via Complexation with (R)-BINOL Tayama, E.; Tanaka, H. Tetrahedron Lett. 2007, 48, 4183-4185. 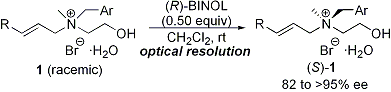 DOI: 10.1016/j.tetlet.2007.04.078 |
| 5 | Regio- and Stereoselective Ferrier Reaction of O-1,3-Dienyl Acetals
Promoted by Organoaluminum Complexes Tayama, E.; Isaka, W. Org. Lett. 2006, 8, 5437-5439. 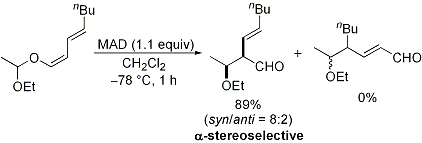 DOI: 10.1021/ol062042j |
| 4 | A Facile and Stereoselective Synthetic Method for Allylic 1,3-Dienyl Ethers Tayama, E.; Sugai, S.; Hara, M. Tetrahedron Lett. 2006, 47, 7533-7535. 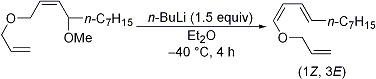 DOI: 10.1016/j.tetlet.2006.08.100 |
| 3 | Asymmetric [1,2] Stevens Rearrangement of (S)-N-Benzylic Proline-Derived Ammonium Salts under Biphasic Conditions Tayama, E.; Nanbara, S.; Nakai, T. Chem. Lett. 2006, 35, 478-479. 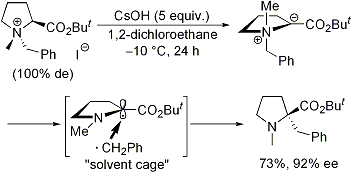 DOI: 10.1246/cl.2006.478 |
| 2 |
A Facile Method for the Stereoselective Preparation of (1Z, 3E)-Dienyl
Ethers via 1,4-Elimination of 1,4-Dialkoxy-(2Z)-alkenes with n-Butyllithium
Tayama, E.; Sugai, S. Synlett 2006, 849-852. 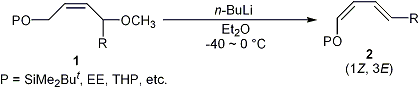 DOI: 10.1055/s-2006-939053 |
| 1 |
Kinetic Resolution via the [2,3] Stevens Rearrangement of Epimeric Six-Membered Ammonium Ylides: A New Entry to Enantio-Enriched α-Amino Acid Derivatives
Tayama, E.; Tanaka, H.; Nakai, T. Heterocycles 2005, 66, 95-99. 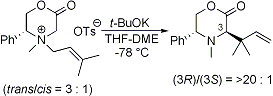 DOI: 10.3987/COM-05-S(K)39 |
その他の論文
| 10 | Solvent-Dependent Reaction Pathways Operating in Copper(II) Tetrafluoroborate Promoted Oxidative Ring-Opening Reactions of Cyclopropyl Silyl Ethers Hasegawa, E.; Nemoto, K.; Nagumo, R.; Tayama, E.; Iwamoto, H. J. Org. Chem. 2016, 81, 2692-2703. |
| 9 | Synthesis of Linear [5]Catenanes via Olefin Metathesis Dimerization of Pseudorotaxanes Composed of a [2]Catenane and a Secondary Ammonium Salt Iwamoto, H.; Tafuku, S.i; Sato, Y.; Takizawa, W.; Katagiri, W.; Tayama, E.; Hasegawa, E.; Fukazawa, Y.; Haino, T. Chem. Commun. 2016, 52, 319-322. |
| 8 | Aryl-Substituted Dimethylbenzimidazolines as Effective Reductants of Photoinduced
Electron Transfer Reactions Hasegawa, E.; Ohta, T.; Tsuji, S.; Mori, K.; Uchida, K.; Miura, T.; Ikoma, T.; Tayama, E.; Iwamoto, H.; Takizawa, S.; Murata, S. Tetrahedron 2015, 71, 5494-5505. |
| 7 |
Metal-Free, One-Pot, Sequential Protocol for Transforming α,β-Epoxy Ketones
to β-Hydroxy Ketones and α-Methylene Ketones
Hasegawa, E.; Arai, S.; Tayama, E.; Iwamoto, H. J. Org. Chem. 2015, 80, 1593-1600. |
| 6 | A Photo-Reagent System of Benzimidazoline and Ru(bpy)3Cl2 to Promote Hexenyl Radical Cyclization and Dowd-Beckwith Ring-Expansion
of α-Halomethyl-Substituted Benzocyclic 1-Alkanones Hasegawa, E.; Tateyama, M.; Hoshi, T.; Ohta, T.; Tayama, E.; Iwamoto, H.; Takizawa, S.; Murata, S. Tetrahedron 2014, 70, 2776-2783. |
| 5 | Carbon-Carbon Bond Formation via Benzoyl Umpolung Attained by Photoinduced
Electron-Transfer with Benzimidazolines Igarashi, T.; Tayama, E.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2013, 54, 6874-6877. |
| 4 | Copper(II)-Salt-Promoted Oxidative Ring-Opening Reactions of Bicyclic Cyclopropanol
Derivatives via Radical Pathways Hasegawa E.; Tateyama, M.; Nagumo, R.; Tayama E.; Iwamoto, H. Beilstein J. Org. Chem. 2013, 9, 1397-1406. |
| 3 | Selective Synthesis of [2]- and [3] Catenane Tuned by Ring Size and Concentration Iwamoto, H.; Takizawa, W.; Itoh, K.; Hagiwara, T.; Tayama, E.; Hasegawa, E.; Haino T. J. Org. Chem. 2013, 78, 5205-5217. |
| 2 | An Effective Procedure to Promote Aza-Prins Cyclization Reactions Employing
a Combination of Ferric Chloride and an Imidazolium Salt in Benzotrifluoride Osawa, C.; Tateyama, M.; Miura, K.; Tayama, E.; Iwamoto, H.; Hasegawa, E. Heterocycles 2012, 86, 1211-1226. |
| 1 | Application of Biphasic Reaction Procedure using Ferric Chloride Dissolved in An Imidazolium Salt and Benzotrifluoride (FeIm-BTF Procedure) to Aza-Prins Cyclization Reaction Hasegawa, E.; Hiroi, N.; Osawa, C.; Tayama, E.; Iwamoto, H. Tetrahedron Lett. 2010, 51, 6535-6538. |
ポスドク時の発表論文
| 3 |
Palladium-Catalyzed Intramolecular Coupling between Aryl Iodides and Allyl
Moieties via Thermal and Microwave-Assisted Conditions
Lautens, M.; Tayama, E.; Herse, C. J. Am. Chem. Soc. 2005, 127, 72-73. |
| 2 |
Activation and Stabilization of Aldimines by an ortho-Trifluoromethyl
Substituent in Direct Vinylogous Mannich-type Reactions
Lautens, M.; Tayama, E.; Nguyen, D. Tetrahedron Lett. 2004, 45, 5131-5133. |
| 1 |
Direct Vinylogous Mannich-Type Reactions via Ring Opening and Rearrangement of Vinyloxiranes
Lautens, M.; Tayama, E.; Nguyen, D. Org. Lett. 2004, 6, 345-347. |
学生時の発表論文
| 11 |
Highly Enantioselective Phase-Transfer-Catalyzed Alkylation of Protected α-Amino
Acid Amides toward Practical Asymmetric Synthesis of Vicinal Diamines, α-Amino
Ketones, and α-Amino Alcohols
Ooi, T.; Takeuchi, M.; Kato, D.; Uematsu, Y.; Tayama, E.; Sakai, D.; Maruoka, K. J. Am. Chem. Soc. 2005, 127, 5073-5083. |
| 10 |
Stereoselective Terminal Functionalization of Small Peptides for Catalytic
Asymmetric Synthesis of Unnatural Peptides
Maruoka, K.; Tayama, E.; Ooi, T. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5824-5829. |
| 9 |
(2,7-Disubstituted-1,8-biphenylenedioxy)bis(dimethylaluminum) as Bidentate Organoaluminum Lewis Acids: Elucidation and Synthetic Utility of the Double Electrophilic Activation Phenomenon
Ooi, T.; Takahashi, M.; Yamada, M.; Tayama, E.; Omoto, K.; Maruoka, K. J. Am. Chem. Soc. 2004, 126, 1150-1160. |
| 8 |
Practical Asymmetric Synthesis of Vicinal Diamines throgh the Catalytic Highly Enantioselective Alkylation of Glycine Amide Derivatives
Ooi, T.; Sakai, D.; Takeuchi, M.; Tayama, E.; Maruoka, K. Angew. Chem. Int. Ed. 2003, 42, 5868-5870. |
| 7 | Highly Stereoselective N-Terminal Functionalization of Small Peptides by Chiral Phase-Transfer Catalysis Ooi, T.; Tayama, E.; Maruoka, K. Angew. Chem. Int. Ed. 2003, 42, 579-582. |
| 6 | Activation of Ether Functionality of Allyl Vinyl Ethers by Chiral Bis(organoaluminum) Lewis Acids: Application to Asymmetric Claisen Rearrangement Tayama, E.; Saito, A.; Ooi, T.; Maruoka, K. Tetrahedron 2002, 58, 8307-8312. |
| 5 | Remarkable Template Effect of a Lewis Acid Receptor in the Intramolecular Radical Cyclization: Control of Reaction Pathway as well as Stereochemistry Ooi, T.; Hokke, Y.; Tayama, E.; Maruoka, K. Tetrahedron 2001, 57, 135-144. |
| 4 | Dramatic Rate Enhancement by Ultrasonic Irradiation in Liquid-Liquid Phase-Transfer Catalytic Reactions Ooi, T.; Tayama, E.; Doda, K.; Takeuchi, M.; Maruoka, K. Synlett 2000, 1500-1502. |
| 3 | Dramatic Enhancement of Reactivity of Organosilicon Compounds Induced by Complexation of Bis(allyl)silanes with Fluoride Ion Shibato, A.; Itagaki, Y.; Tayama, E.; Hokke, Y.; Asao, N.; Maruoka, K. Tetrahedron 2000, 56, 5373-5382. |
| 2 | Efficient Synthesis of Aromatic sec-Amides from Esters: Synthetic Utility
of Dilithium Amides Ooi, T.; Tayama, E.; Yamada, M.; Maruoka, K. Synlett 1999, 729-730. |
| 1 | Bidentate organoaluminum Lewis acid for selective activation of carbonyl
over acetal functionality: Chemoselective functionalization Ooi, T.; Tayama, E.; Takahashi, M.; Maruoka, K. Tetrahedron Lett. 1997, 38, 7403-7406 |
受賞等
2004年 有機合成化学協会研究企画賞(第一製薬)
|
