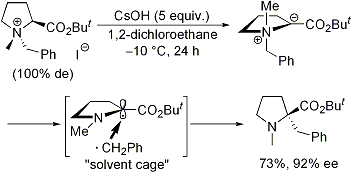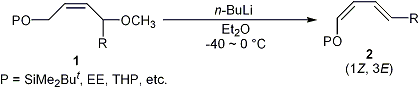Go Japanese Page
Dr. Eiji Tayama Home Page
(Last updated 2025.9.24)
Our group is not able to employ postdoctoral applicants at this time.
About Eiji Tayama

Eiji Tayama(田山 英治)
Associate Professor of Niigata University
Department of Chemistry
Faculty of Science
Niigata University
E-Mail: tayama[at]chem.sc.niigata-u.ac.jp
Academic Positions
2008-Present Associate Professor, Niigata University
2003-2008 Assistant Professor, Niigata University
Education
2002-2003 Toronto University (CANADA), Post-doctoral fellow with Prof. Mark Lautens
1997-2002 Hokkaido University, Ph.D. with Prof. Keiji Maruoka
1993-1997 Utsunomiya University, B.Sc.
Areas of Research Interest
The Study of N-Chiral Nitrogen Compounds
Rearrangement Reactions
Elimination reactions
Development of New Synthetic Methods
Synthesis of Unnatural Amino Acid Derivatives
Link
Niigata University
Faculty of Science
Publications
Dr. Eiji Tayama Home Page
(Last updated 2025.9.24)
Our group is not able to employ postdoctoral applicants at this time.
About Eiji Tayama

Eiji Tayama(田山 英治)
Associate Professor of Niigata University
Department of Chemistry
Faculty of Science
Niigata University
E-Mail: tayama[at]chem.sc.niigata-u.ac.jp
Academic Positions
2008-Present Associate Professor, Niigata University
2003-2008 Assistant Professor, Niigata University
Education
2002-2003 Toronto University (CANADA), Post-doctoral fellow with Prof. Mark Lautens
1997-2002 Hokkaido University, Ph.D. with Prof. Keiji Maruoka
1993-1997 Utsunomiya University, B.Sc.
Areas of Research Interest
The Study of N-Chiral Nitrogen Compounds
Rearrangement Reactions
Elimination reactions
Development of New Synthetic Methods
Synthesis of Unnatural Amino Acid Derivatives
Link
Niigata University
Faculty of Science
Publications
| 71 | Paper Ring-Strain Effects in Sommelet-Hauser Rearrangement of Five- and Six-Membered Fused Bicyclic Ammonium Ylides Tayama, E.; Itoyama, K.; Sasazaki, J. Synthesis 2025, in press. 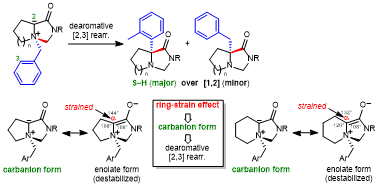 DOI: 10.1055/a-2707-7636 |
| 70 | Full Review Nitrogen- and Sulfur-Based Stevens and Related Rearrangements Tayama, E. Comprehensive Organic Synthesis, 3rd Ed.,Vol. 3, Chap. 4.14; Molander, G.; Knochel, P., Ed.; Elsevier: 2025, 677-747. 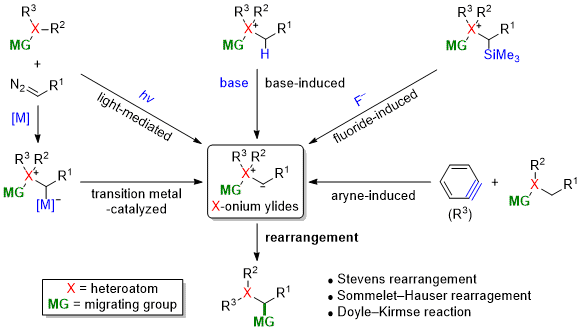 DOI: 10.1016/B978-0-323-96025-0.00029-6 |
| 69 | Research Article Controlling the position of the nucleophilic ring-opening of 2-EWG-substituted azetidinium salts with fluoride by the N-1-(1-naphthyl)ethyl substituent and BINAM-derived bis-urea organocatalyst Tayama, E.; Tsutsumi, R.; Uraguchi, D. Tetrahedron 2024, 167, 134274. 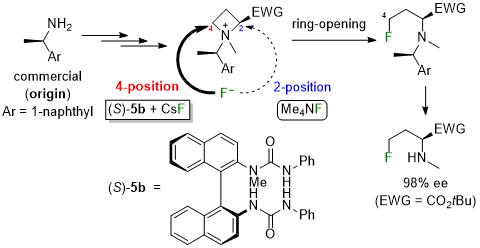 DOI: 10.1016/j.tet.2024.134274 |
| 68 | Research Article Synthesis of α-Fluoro-γ-aminobutyric Acid Derivatives by Nucleophilic Ring-opening of Azetidine-2-carboxylic Acid-derived Ammonium Salts with Fluoride Tayama, E.; Kurosaki, Y.; Iida, M.; Aida, T.; Nakanome, N. Tetrahedron 2023, 142, 133528. 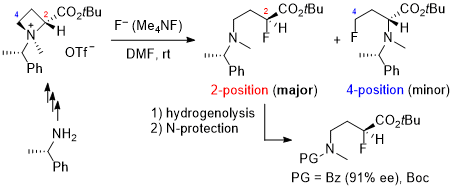 DOI: 10.1016/j.tet.2023.133528 |
| 67 | Short Review Recent Advances in the Generation of Onium Ylides for Sommelet-Hauser Rearrangements Tayama, E. Synthesis 2022, 54, 5385. 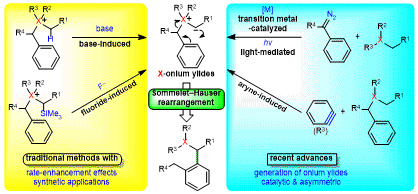 DOI: 10.1055/a-1914-7261 |
| 66 | Research Article Chiral Tetraalkynylborate as a Chiral Solvating Agent for N-Chiral Tetraalkylammonium Salts Tayama, E.; Nishio, R. Tetrahedron 2022, 114, 132783. 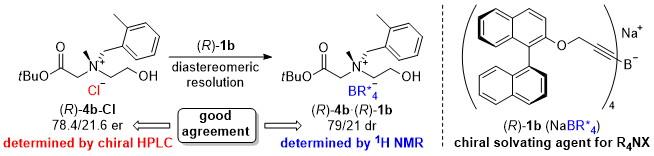 DOI: 10.1016/j.tet.2022.132783 |
| 65 | Research Article Base-Induced Sommelet–Hauser Rearrangement of N-(Pyridinylmethyl) Tetraalkylammonium Salts Tayama, E.; Shimizu, G.; Nakao R. Tetrahedron 2022, 111, 132721. 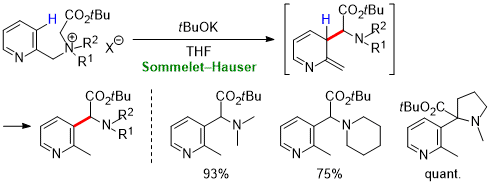 DOI: 10.1016/j.tet.2022.132721 |
| 64 | Synthesis of Tertiary Alkyl Fluorides and Chlorides by Site-Selective Nucleophilic
Ring-Opening Reaction of α-Aryl Azetidinium Salts Tayama, E.; Kawai, K. RSC Adv. 2021, 11, 39607-39618. 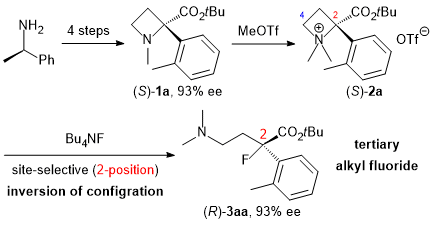 DOI: 10.1039/D1RA08706A ※Open Access |
| 63 | Synthesis of Optically Active 2-Substituted Azetidine-2-carbonitriles from Chiral 1-Arylethylamine via α-Alkylation of N-Borane Complexes Tayama, E.; Nakanome, N. RSC Adv. 2021, 11, 23825-23837. 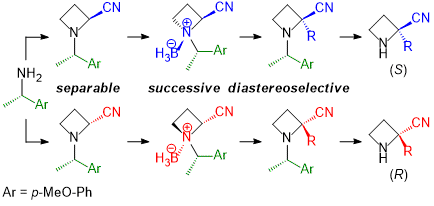 DOI: 10.1039/D1RA04585G ※Open Access |
| 62 | Brønsted Acid-Catalyzed Aza-Ferrier Reaction of N,O-Allenyl Acetals: Synthesis
of β-Amino-α-methylene Aldehydes Tayama, E.; Ishikawa, Y. J. Org. Chem. 2020, 85, 9405-9414. 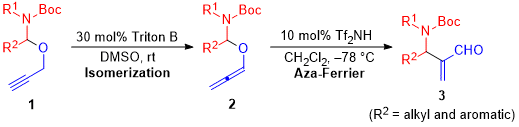 DOI: 10.1021/acs.joc.0c01047 |
| 61 | Base-induced Sommelet-Hauser Rearrangement of N-(α-(2-Oxyethyl)branched)benzylic
Glycine Ester-derived Ammonium Salts via a Chelated Intermediate Tayama, E.; Hirano, K.; Baba, S. Tetrahedron 2020, 76, 131064. 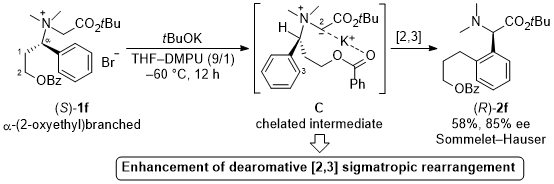 DOI: 10.1016/j.tet.2020.131064 |
| 60 | Base-promoted Aromatic [3,3] Sigmatropic Rearrangement of N-Acyl-O-arylhydroxylamine
Derivatives Tayama, E.; Hirano, K. Tetrahedron 2019, 75, 665-673.  DOI: 10.1016/j.tet.2018.12.058 |
| 59 | Chiral Tetraaryl- and Tetraalkynylborates as Chiral Solvating Agents for Tetraalkylammonium Salts Tayama, E.; Sugawara, T. Eur. J. Org. Chem. 2019, 803-811. 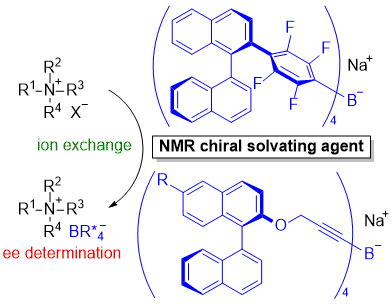 DOI: 10.1002/ejoc.201801448 |
| 58 | Base-promoted Diastereoselective α-Alkylation of Borane N-((S)-1’-Phenylethyl)azetidine-2-carboxylic
acid Ester Complexes Tayama, E.; Nishio, R.; Kobayashi, Y. Org. & Biomol. Chem. 2018, 16, 5833-5845. 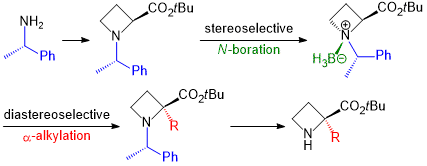 DOI: 10.1039/C8OB01395K |
| 57 | Dearomative [2,3] Sigmatropic Rearrangement of Ammonium Ylides Followed
by 1,4-Elimination to Form α-(ortho-Vinylphenyl)Amino Acid Esters Tayama, E.; Sotome, S. Org. & Biomol. Chem. 2018, 16, 4833-4839. 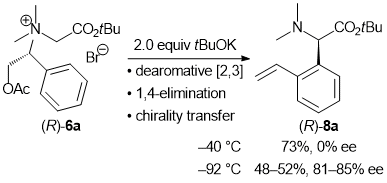 DOI: 10.1039/C8OB01091A |
| 56 | Structural and Mechanistic Studies of the Base-Induced Sommelet-Hauser
Rearrangement of N-α-Branched Benzylic Azetidine-2-carboxylic Acid-Derived
Ammonium Salts Tayama, E.; Watanabe, K.; Sotome, S. Org. & Biomol. Chem. 2017, 15, 6668-6678. 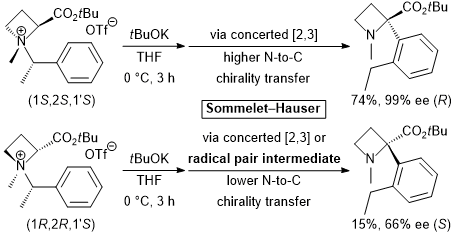 DOI: 10.1039/C7OB01391D |
| 55 | Diaza [1,4] Wittig-Type Rearrangement of N-Allylic-N-Boc-hydrazines into
γ-Amino-N-Boc-Enamines Tayama, E.; Kobayashi, Y.; Toma, Y. Chem. Commun. 2016, 52, 10570-10573. 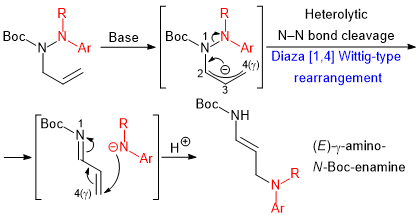 DOI: 10.1039/C6CC04626F |
| 54 | Ring-Strain Effect in Base-Induced Sommelet-Hauser Rearrangement: Application
to Successive Stereo-Controlled Transformations Tayama, E.; Watanabe, K.; Matano, Y. Eur. J. Org. Chem. 2016, 3631-3641. 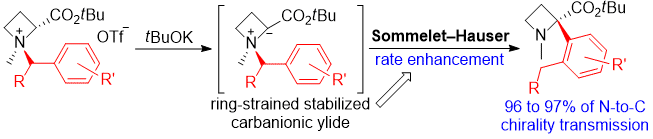 DOI: 10.1002/ejoc.201600611 |
| 53 | Review Ring-Substitution, Enlargement, and Contraction by Base-Induced Rearrangements of N-Heterocyclic Ammonium Salts Tayama, E. Heterocycles 2016, 92, 793-828. 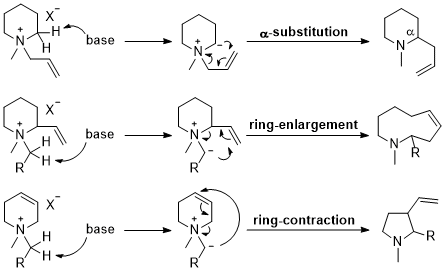 DOI: 10.3987/REV-16-837 |
| 52 | Solvent-Dependent Reaction Pathways Operating in Copper(II) Tetrafluoroborate
Promoted Oxidative Ring-Opening Reactions of Cyclopropyl Silyl Ethers Hasegawa, E.; Nemoto, K.; Nagumo, R.; Tayama, E.; Iwamoto, H. J. Org. Chem. 2016, 81, 2692-2703. |
| 51 | Regioselective Synthesis of Secondary 1,3-Dienamides by Successive Eliminations Tayama, E.; Saito, S. Tetrahedron 2016, 72, 599-604. 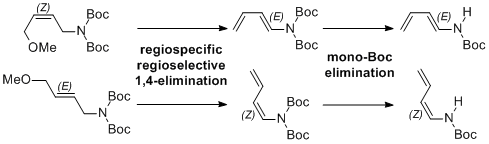 DOI: 10.1016/j.tet.2015.11.065 |
| 50 | Synthesis of Linear [5]Catenanes via Olefin Metathesis Dimerization of Pseudorotaxanes Composed of a [2]Catenane and a Secondary Ammonium Salt Iwamoto, H.; Tafuku, S.i; Sato, Y.; Takizawa, W.; Katagiri, W.; Tayama, E.; Hasegawa, E.; Fukazawa, Y.; Haino, T. Chem. Commun. 2016, 52, 319-322. |
| 49 | Personal Account Recent Advances in the Base-Induced Sommelet-Hauser Rearrangement of Amino Acid-Derived Ammonium Ylides Tayama, E. Chem. Rec. 2015, 789-800. 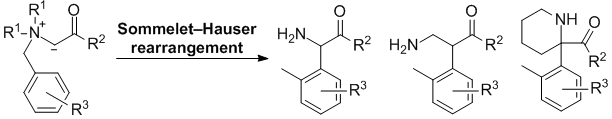 DOI: 10.1002/tcr.201500009 |
| 48 | Copper-Catalyzed Regiospecific and 1,2-Regioselective Cyclopropanation
of (1Z)-1-Amino- and (1Z)-1-Oxy-1,3-Butadienyl Derivatives Tayama, E.; Saito, S. Synlett 2015, 1880-1884. 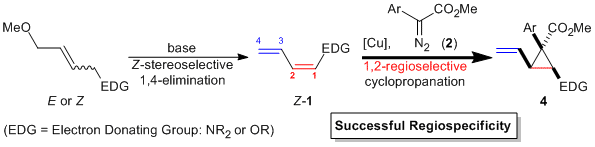 DOI: 10.1055/s-0034-1380426 |
| 47 | Aryl-Substituted Dimethylbenzimidazolines as Effective Reductants of Photoinduced
Electron Transfer Reactions Hasegawa, E.; Ohta, T.; Tsuji, S.; Mori, K.; Uchida, K.; Miura, T.; Ikoma, T.; Tayama, E.; Iwamoto, H.; Takizawa, S.; Murata, S. Tetrahedron 2015, 71, 5494-5505. |
| 46 |
Metal-Free, One-Pot, Sequential Protocol for Transforming α,β-Epoxy Ketones
to β-Hydroxy Ketones and α-Methylene Ketones
Hasegawa, E.; Arai, S.; Tayama, E.; Iwamoto, H. J. Org. Chem. 2015, 80, 1593-1600. |
| 45 | Stereoselective Preparation of (1Z)- and (1E)-N-Boc-1-Amino-1,3-Dienes
by Stereospecific Base-Promoted 1,4-Elimination Tayama, E.; Toma, Y. Tetrahedron 2015, 71, 554-559. 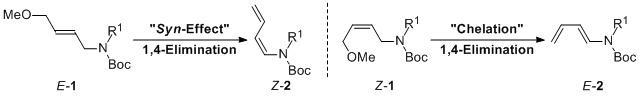 DOI: 10.1016/j.tet.2014.12.039 |
| 44 | Double Axial Chirality Promoted Asymmetric [2,3] Stevens Rearrangement
of N-Cinnamyl L-Alanine Amide-Derived Ammonium Ylides Tayama, E.; Naganuma, N.; Iwamoto, H.; Hasegawa, E. Chem. Commun. 2014, 50, 6860-6862. 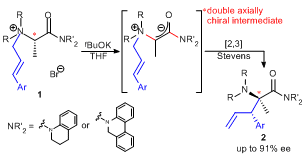 DOI: 10.1039/C4CC02536A |
| 43 | Copper(II)-Acid Catalyzed Cyclopropanation of 1,3-Dienamides by Electrophilic
Activation of α-Aryl Diazoesters Tayama, E.; Horikawa, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2014, 55, 3041-3044. 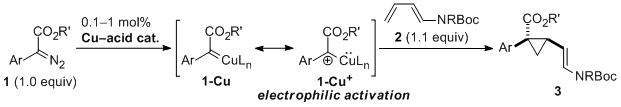 DOI: 10.1016/j.tetlet.2014.03.115 |
| 42 | Personal Account New Development of Base-Induced Sommele-Hauser Rearrangement of Ammonium Ylides Tayama, E. J. Synth. Org. Chem., Japan, 2014, 72, 418-428. 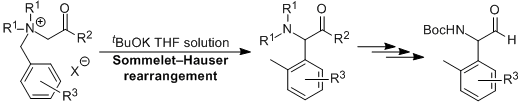 DOI: 10.5059/yukigoseikyokaishi.72.418 |
| 41 | A Photo-Reagent System of Benzimidazoline and Ru(bpy)3Cl2 to Promote Hexenyl Radical Cyclization and Dowd-Beckwith Ring-Expansion
of α-Halomethyl-Substituted Benzocyclic 1-Alkanones Hasegawa, E.; Tateyama, M.; Hoshi, T.; Ohta, T.; Tayama, E.; Iwamoto, H.; Takizawa, S.; Murata, S. Tetrahedron 2014, 70, 2776-2783. |
| 40 | Carbon-Carbon Bond Formation via Benzoyl Umpolung Attained by Photoinduced
Electron-Transfer with Benzimidazolines Igarashi, T.; Tayama, E.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2013, 52, 1819-1821. |
| 39 | Copper(II)-Salt-Promoted Oxidative Ring-Opening Reactions of Bicyclic Cyclopropanol
Derivatives via Radical Pathways Hasegawa E.; Tateyama, M.; Nagumo, R.; Tayama E.; Iwamoto, H. Beilstein J. Org. Chem. 2013, 9, 1397-1406. |
| 38 | Selective Synthesis of [2]- and [3] Catenane Tuned by Ring Size and Concentration Iwamoto, H.; Takizawa, W.; Itoh, K.; Hagiwara, T.; Tayama, E.; Hasegawa, E.; Haino T. J. Org. Chem. 2013, 78, 5205-5217. |
| 37 | 1,4-Elimination/Bronsted Acid Catalyzed Aza-Ferrier Reaction Sequence as
an Entry to β-Amino-β,γ-Unsaturated Aldehydes Tayama, E.; Horikawa, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron 2013, 69, 2745-2752. 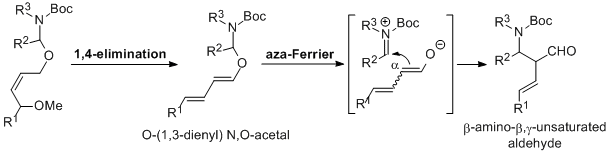 DOI: 10.1016/j.tet.2013.01.088 |
| 36 | A Unique and Simple Preparative Method for α-Arylpipecolinic Acid Esters
via Base-induced Sommelet-Hauser Rearrangement Tayama, E.; Sato, R.; Ito, M.; Iwamoto, H.; Hasegawa, E. Heterocycles 2013, 87, 381-388. 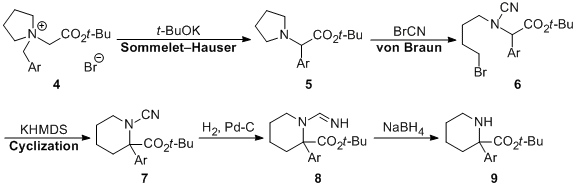 DOI: 10.3987/COM-12-12619 |
| 35 | An Effective Procedure to Promote Aza-Prins Cyclization Reactions Employing
a Combination of Ferric Chloride and an Imidazolium Salt in Benzotrifluoride Osawa, C.; Tateyama, M.; Miura, K.; Tayama, E.; Iwamoto, H.; Hasegawa, E. Heterocycles 2012, 86, 1211-1226. DOI: 10.3987/COM-12-S(N)78 |
| 34 | Copper(II)-Acid Co-Catalyzed Intermolecular Substitution of Electron-Rich
Aromatics with Diazoesters Tayama, E.; Ishikawa, M.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2012, 53, 5159-5161. 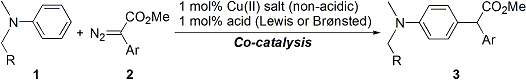 DOI: 10.1016/j.tetlet.2012.07.070 |
| 33 | A Formal Method for the De-N,N-Dialkylation of Sommelet-Hauser Rearrangement
Products Tayama, E.; Sato, R.; Takedachi, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron 2012, 68, 4710-4718. 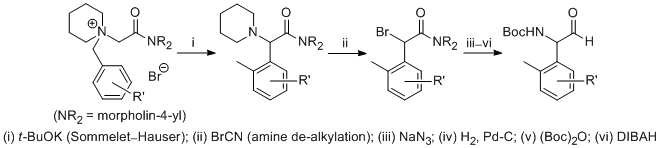 DOI: 10.1016/j.tet.2012.04.015 |
| 32 | 1,2-Dimethoxy-4,5-dimethylene: A New Protecting Group for Acyclic Amino Acid Derivatives Prepared by Stevens Rearrangement Tayama, E.; Takedachi, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2012, 53, 1373-1375. 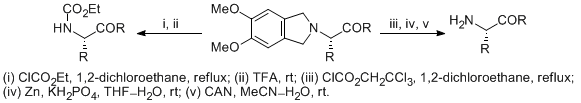 DOI: 10.1016/j.tetlet.2012.01.013 |
| 31 | Asymmetric α-2-Tosylethenylation of N,N-Dialkyl-L-Amino Acid Esters via the Formation of Non-Racemic Ammonium Enolates Tayama, E.; Igarashi, T.; Iwamoto, H.; Hasegawa, E. Org. Biomol. Chem. 2012, 10, 339-345. 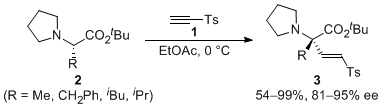 DOI: 10.1039/C1OB06074K |
| 30 | Asymmetric α-2-Tosylvinylation of in situ-generated N-2-Tosylvinyl Proline-Derived Ammonium Ylides Igarashi, T.; Tayama, E.; Iwamoto, H.; Hasegawa, E. Tetrahedron Lett. 2011, 52, 1819-1821. 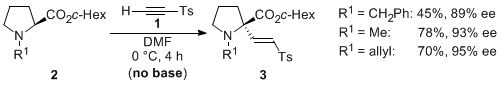 DOI: 10.1016/j.tetlet.2011.02.040 |
| 29 | Copper(II) Triflate Catalyzed Intermolecular Aromatic Substitution of N,N-Disubstituted
Anilines with Diazoesters Tayama, E.; Yanaki, T.; Iwamoto, H.; Hasegawa, E. Eur. J. Org. Chem. 2010, 6719-6721. 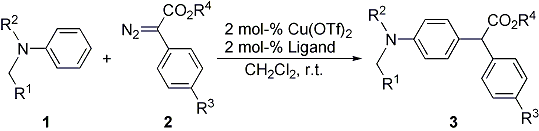 DOI: 10.1002/ejoc.201001083 |
| 28 | Application of Biphasic Reaction Procedure using Ferric Chloride Dissolved in An Imidazolium Salt and Benzotrifluoride (FeIm-BTF Procedure) to Aza-Prins Cyclization Reaction Hasegawa, E.; Hiroi, N.; Osawa, C.; Tayama, E.; Iwamoto, H. Tetrahedron Lett. 2010, 51, 6535-6538. |
| 27 | Remarkable Enhancement Effect of Potassium tert-Butoxide/THF Solution in
Base-induced Sommelet-Hauser Rearrangements Tayama, E.; Takedachi, K.; Iwamoto, H.; Hasegawa, E. Tetrahedron 2010, 66, 9389-9395. 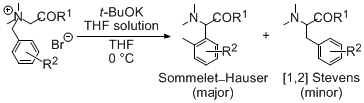 DOI: 10.1016/j.tet.2010.09.105 |
| 26 | Resolution of Nitrogen-Centered Chiral Tetraalkylammonium Salts: Application
to [1,2] Stevens Rearrangements with N-to-C Chirality Transmission Tayama, E.; Otoyama, S.; Tanaka, H. Tetrahedron: Asymmetry 2009, 20, 2600-2608. 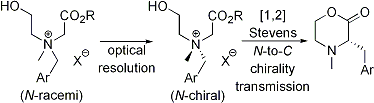 DOI: 10.1016/j.tetasy.2009.10.025 |
| 25 | New Synthetic Routes to Optically Active α-Quaternary α-Aryl Amino Acid Derivatives via the Diastereoselective Stevens and Sommelet-Hauser Rearrangements Tayama, E.; Orihara, K.; Kimura, H. Org. & Biomol. Chem. 2008, 6, 3673-3680. 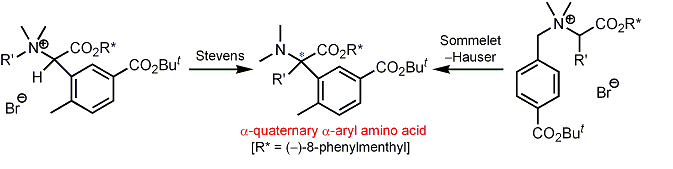 DOI: 10.1039/b811162f |
| 24 | Bronsted Acid Catalyzed Regioselective Aza-Ferrier Reaction: A Novel Synthetic Method of α-(N-Boc-2-pyrrolidinyl)aldehydes Tayama, E.; Otoyama, S.; Isaka W. Chem. Comm. 2008, 4216-4218. 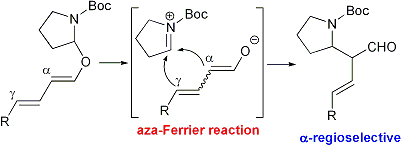 DOI: 10.1039/b806492j |
| 23 | Asymmetric Sommelet-Hauser Rearrangement of N-Benzylic Ammonium Salts Tayama, E.; Kimura, H. Angew. Chem. Int. Ed. 2007, 46, 8869-8871. 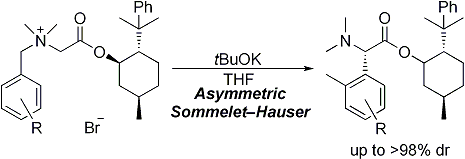 DOI: 10.1002/anie.200703832 |
| 22 | A Facile Synthetic Method of α-Quaternary-β,γ-Unsaturated Aldehydes via the Stereoselective 1,4-Elimination and α-Regioselective Ferrier Reaction Tayama, E.; Hashimoto, R. Tetrahedron Lett. 2007, 48, 7950-7952. 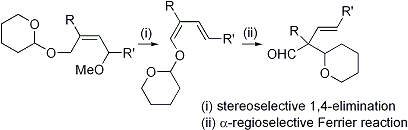 DOI: 10.1016/j.tetlet.2007.09.049 |
| 21 | A Facile Method for the Stereoselective Preparation of (1E,3E)-4-Substituted-1-Amino-1,3-Dienes
via 1,4-Elimination Tayama, E.; Sugai, S. Tetrahedron Lett. 2007, 48, 6163-6166. 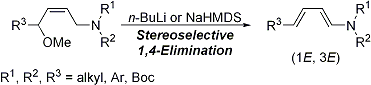 DOI: 10.1016/j.tetlet.2007.06.140 |
| 20 | An Efficient Optical Resolution of Nitrogen-Centered Chiral β-Hydroxy-Tetraalkylammonium
Salts via Complexation with (R)-BINOL Tayama, E.; Tanaka, H. Tetrahedron Lett. 2007, 48, 4183-4185. 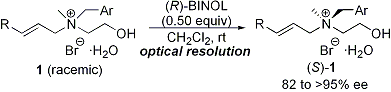 DOI: 10.1016/j.tetlet.2007.04.078 |
| 19 | Regio- and Stereoselective Ferrier Reaction of O-1,3-Dienyl Acetals
Promoted by Organoaluminum Complexes Tayama, E.; Isaka, W. Org. Lett. 2006, 8, 5437-5439. 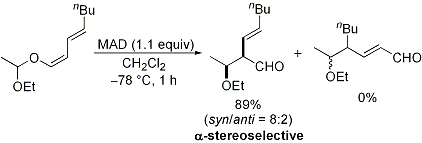 DOI: 10.1016/j.tetlet.2007.04.078 |
| 18 | A Facile and Stereoselective Synthetic Method for Allylic 1,3-Dienyl Ethers Tayama, E.; Sugai, S.; Hara, M. Tetrahedron Lett. 2006, 47, 7533-7535. 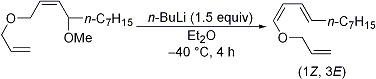 DOI: 10.1016/j.tetlet.2006.08.100 |
| 17 |
Asymmetric [1,2] Stevens Rearrangement of (S)-N-Benzylic Proline-Derived
Ammonium Salts under Biphasic Conditions
Tayama, E.; Nanbara, S.; Nakai, T. Chem. Lett. 2006, 35, 478-479. |
| 16 |
A Facile Method for the Stereoselective Preparation of (1Z,3E)-Dienyl Ethers
via 1,4-Elimination of 1,4-Dialkoxy-(2Z)-alkenes with n-Butyllithium
Tayama, E.; Sugai, S. Synlett 2006, 849-852. |
| 15 |
Kinetic Resolution via the [2,3] Stevens Rearrangement of Epimeric Six-Membered
Ammonium Ylides: A New Entry to Enantio-Enriched α-Amino Acid Derivatives
Tayama, E.; Tanaka, H.; Nakai, T. Heterocycles 2005, 66, 95-99. 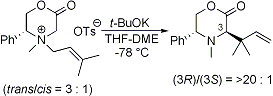 DOI: 10.3987/COM-05-S(K)39 |
| 14 |
Highly Enantioselective Phase-Transfer-Catalyzed Alkylation of Protected α-Amino
Acid Amides toward Practical Asymmetric Synthesis of Vicinal Diamines, α-Amino
Ketones, and a-Amino Alcohols
Ooi, T.; Takeuchi, M.; Kato, D.; Uematsu, Y.; Tayama, E.; Sakai, D.; Maruoka, K. J. Am. Chem. Soc. 2005, 127, 5073-5083. |
| 13 |
Palladium-Catalyzed Intramolecular Coupling between Aryl Iodides and Allyl Moieties via Thermal and Microwave-Assisted Conditions
Lautens, M.; Tayama, E.; Herse, C. J. Am. Chem. Soc. 2005, 127, 72-73. |
| 12 |
Activation and Stabilization of Aldimines by an ortho-Trifluoromethyl
Substituent in Direct Vinylogous Mannich-type Reactions
Lautens, M.; Tayama, E.; Nguyen, D. Tetrahedron Lett. 2004, 45, 5131-5133. |
| 11 |
Stereoselective Terminal Functionalization of Small Peptides for Catalytic Asymmetric Synthesis of Unnatural Peptides
Maruoka, K.; Tayama, E.; Ooi, T. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5824-5829. |
| 10 |
(2,7-Disubstituted-1,8-biphenylenedioxy)bis(dimethylaluminum) as Bidentate
Organoaluminum Lewis Acids: Elucidation and Synthetic Utility of the
Double Electrophilic Activation Phenomenon
Ooi, T.; Takahashi, M.; Yamada, M.; Tayama, E.; Omoto, K.; Maruoka, K. J. Am. Chem. Soc. 2004, 126, 1150-1160. |
| 9 |
Direct Vinylogous Mannich-Type Reactions via Ring Opening and Rearrangement
of Vinyloxiranes
Lautens, M.; Tayama, E.; Nguyen, D. Org. Lett. 2004, 6, 345-347. |
| 8 |
Practical Asymmetric Synthesis of Vicinal Diamines throgh the Catalytic
Highly Enantioselective Alkylation of Glycine Amide Derivatives
Ooi, T.; Sakai, D.; Takeuchi, M.; Tayama, E.; Maruoka, K. Angew. Chem. Int. Ed. 2003, 42, 5868-5870. |
| 7 | Highly Stereoselective N-Terminal Functionalization of Small Peptides by Chiral Phase-Transfer Catalysis Ooi, T.; Tayama, E.; Maruoka, K. Angew. Chem. Int. Ed. 2003, 42, 579-582. |
| 6 | Activation of Ether Functionality of Allyl Vinyl Ethers by Chiral Bis(organoaluminum) Lewis Acids: Application to Asymmetric Claisen Rearrangement Tayama, E.; Saito, A.; Ooi, T.; Maruoka, K. Tetrahedron 2002, 58, 8307-8312. |
| 5 | Remarkable Template Effect of a Lewis Acid Receptor in the Intramolecular Radical Cyclization: Control of Reaction Pathway as well as Stereochemistry Ooi, T.; Hokke, Y.; Tayama, E.; Maruoka, K. Tetrahedron 2001, 57, 135-144. |
| 4 | Dramatic Rate Enhancement by Ultrasonic Irradiation in Liquid-Liquid Phase-Transfer Catalytic Reactions Ooi, T.; Tayama, E.; Doda, K.; Takeuchi, M.; Maruoka, K. Synlett 2000, 1500-1502. |
| 3 | Dramatic Enhancement of Reactivity of Organosilicon Compounds Induced by Complexation of Bis(allyl)silanes with Fluoride Ion Shibato, A.; Itagaki, Y.; Tayama, E.; Hokke, Y.; Asao, N.; Maruoka, K. Tetrahedron 2000, 56, 5373-5382. |
| 2 | Efficient Synthesis of Aromatic sec-Amides from Esters: Synthetic Utility of Dilithium Amides Ooi, T.; Tayama, E.; Yamada, M.; Maruoka, K. Synlett 1999, 729-730. |
| 1 | Bidentate organoaluminum Lewis acid for selective activation of carbonyl over acetal functionality: Chemoselective functionalization Ooi, T.; Tayama, E.; Takahashi, M.; Maruoka, K. Tetrahedron Lett. 1997, 38, 7403-7406 |